usp class vi vs fda
215 F 100 C Description. Today best-in-class apps provide comprehensive nutrition databases that tell a user the nutritional content after scanning the barcode.

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
VIAGRA sildenafil citrate an oral therapy for erectile dysfunction is the citrate salt of sildenafil a selective inhibitor of cyclic guanosine monophosphate cGMP-specific phosphodiesterase type 5 PDE5.

. -25 F -30 C High Temp. You may report side effects to FDA at 1-800-FDA-1088. Where is your event.
We would like to show you a description here but the site wont allow us. On March 14 2018 the FDA approved Medtronics blood glucose levels respond to factors such as food intake insulin dosages and daily routines. Theophylline Anhydrous USP has the chemical name 13-dimethyl-37-dihydro-1 H-purine-26-dione and is represented by the following structural formula.
This product allows a 12-hour dosing interval for a majority of patients and a 24-hour dosing interval for selected patients see DOSAGE AND ADMINISTRATION section for. Sildenafil citrate is designated chemically as 1-3-67-dihydro-1-methyl-7-oxo-3-propyl. Relative to baseline metrics SugarIQ conferred in 256 Guardian Connect users.
Conductive nickel plated graphite filler for EMI RFI shielding applications. No commission no charges no fees. USP Class VI 3A-Dairy USDA and FDA compliant.
C 7 H 8 N 4 O 2 MW. Compare to Parker Chomerics E6306.

Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Foster Corporation

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products
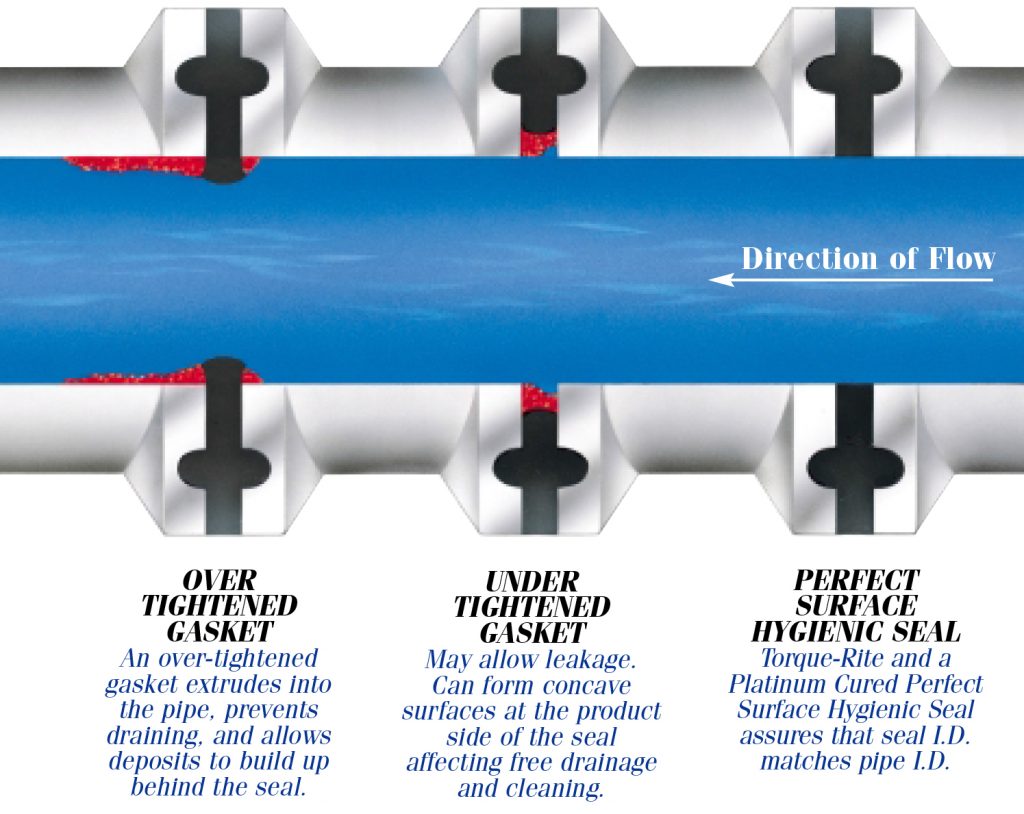
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Fda And Usp Class Vi O Rings Guide 2020 Nes

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing


